
Introduction: A Simple Question and a Complex Answer
Fuel prices are rising. Emission standards are getting stricter. And everyone wants a clean engine that doesn’t feel like a compromise. That naturally raises a big question: Can we use hydrogen in place of CNG in cars?
At first glance, the idea seems almost perfect. Hydrogen burns cleanly. It has no carbon. Rockets use it. So why not cars?
The short answer is: Yes, hydrogen can work in cars – but not as easily as CNG.
The long answer lies in understanding how hydrogen behaves/works inside an engine.
Let’s break it down step by step, without hype, without false claims, and with real engineering logic.
How Hydrogen Combustion Engines Really Work
Most people assume that hydrogen engines require completely new technology. That’s not true.
A hydrogen combustion engine works on the same basic principle as a petrol or CNG engine. It uses the same four strokes:
- Intake
- Compression
- Power
- Exhaust
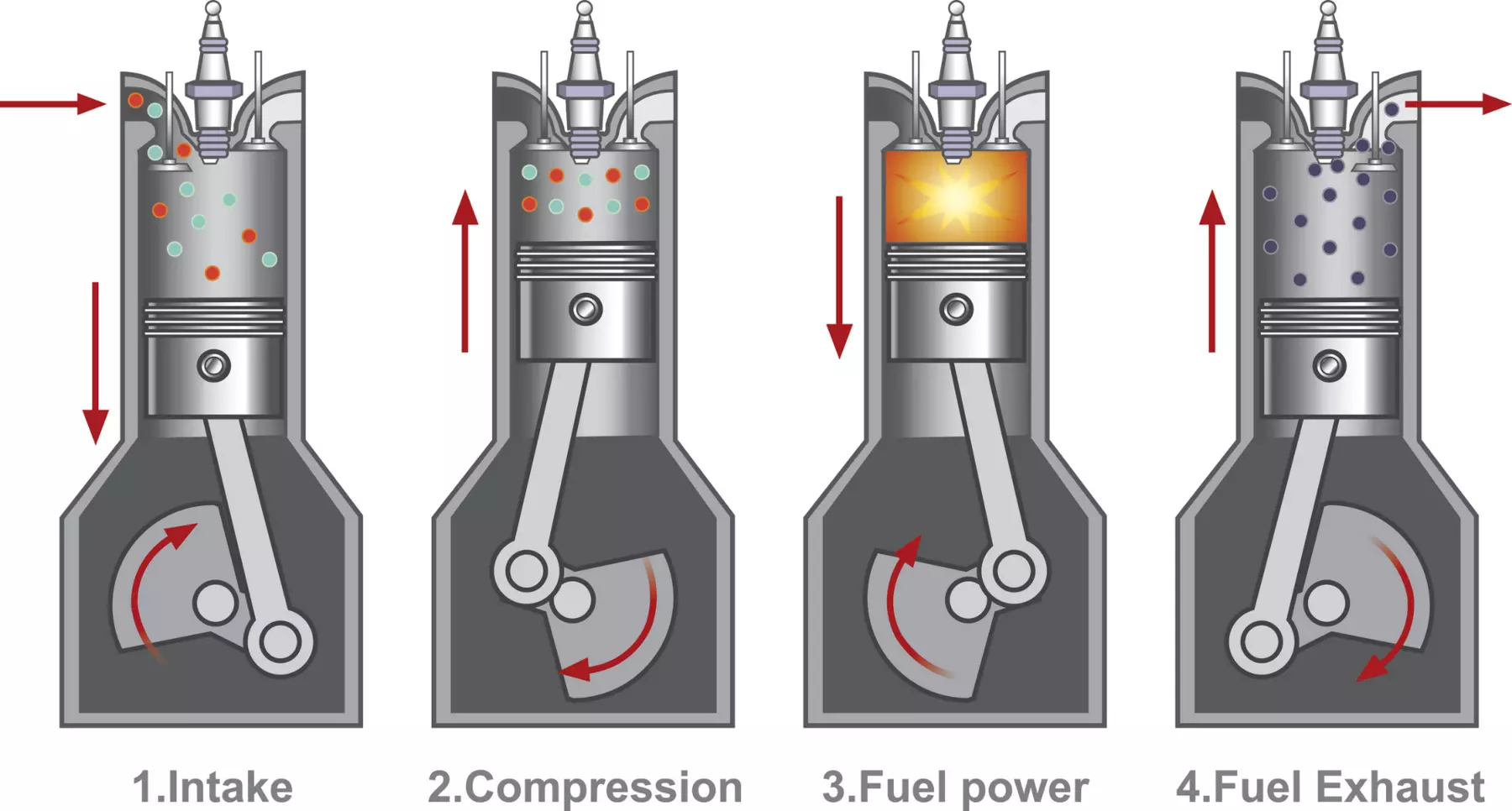
In fact, if you understand how a petrol engine works, you already understand the basics of a hydrogen engine.
The main difference is that there is fuel, not an engine cycle.
When hydrogen enters the combustion chamber, it behaves very differently from petrol or CNG, and that changes everything.
Hydrogen combustion: clean, but not perfect
The chemistry of hydrogen combustion is very simple.
When hydrogen burns with oxygen, the by-product is water.
Hydrogen + Oxygen → Water
No carbon. No CO₂.
In theory, this makes hydrogen a zero-carbon fuel.
But in theory, real engines don’t work.
Inside a running engine, the combustion temperature rises rapidly. At that high temperature, nitrogen from the intake air reacts with oxygen, forming NOx (nitrogen oxides).
So in practice, the reaction looks like this:
Hydrogen + oxygen + nitrogen → water + NOx
This makes hydrogen engines carbon-free, but not completely emission-free.
That still puts hydrogen ahead of petrol and CNG in terms of carbon emissions, but that doesn’t make it a magic bullet.
How is this different from petrol and CNG ?
Petrol is a hydrocarbon fuel. It contains both carbon and hydrogen.
When petrol burns, it produces CO₂, water and other harmful emissions.
CNG also contains carbon, although it burns much cleaner than petrol.
Hydrogen completely removes carbon from the equation. This is its biggest environmental advantage, and the main reason why engineers continue to explore it.
Air-fuel ratio: Hydrogen’s big advantage
A strong advantage of hydrogen lies in its air-fuel ratio.
The benchmark here is the stoichiometric air-fuel ratio, the exact ratio where the fuel burns completely, leaving no excess air or fuel.
Here’s a comparison of the figures:
- Petrol: ~14.7:1
- CNG: ~17.2:1
- Hydrogen: ~34:1
A higher air-fuel ratio means the engine uses more air and less fuel.
It brings with it multiple benefits:
- More complete combustion
- Better fuel efficiency
- Lower combustion temperature
- Reduced heat loss
Hydrogen can also burn in extremely lean mixtures.
- Hydrogen can burn at lean ratios as high as 180:1
- Petrol engines struggle at more than 18:1
- CNG engines operate at around 22:1
This flexibility gives hydrogen a huge efficiency advantage.
Lean Mixtures Mean Less Power
As the mixture becomes leaner, power output drops. While hydrogen engines are very efficient, they can feel underpowered, especially when compared to petrol engines.
Ignition energy: Hydrogen is very easy to burn
Hydrogen requires very little ignition energy to burn.
To put this into perspective:
- A petrol-air mixture requires about 0.24 millijoules of energy
- A hydrogen-air mixture requires only 0.02 millijoules of energy
That makes hydrogen incredibly easy to ignite.
Why this is good
- Cold starts are easier
- Even a weak spark can start combustion
Why this is dangerous
- Hot spots inside the engine can cause pre-ignition
- If ignition control is poor, misfires are more likely
Simply put: hydrogen forgives a weak spark but punishes poor control.
This makes ignition timing and thermal management absolutely critical.
Hydrogen mixes faster than other fuels
Hydrogen diffuses very quickly in air.
This rapid diffusion helps create a uniform air-fuel mixture inside the cylinder, especially in direct injection systems.
Uniform mixing:
- Improves combustion stability
- Reduces incomplete combustion
- Supports lean operation
This property works strongly in favor of hydrogen from an engineering standpoint.
Flame velocity: Faster fuel, higher efficiency
Hydrogen has a much higher flame velocity than gasoline.
This means that combustion ends quickly, and pressure builds up near the top dead center (TDC) of the piston.
This is where the engine does its most useful work.
Benefits of a faster flame velocity:
- Higher thermal efficiency
- Better high-RPM performance
- More effective power extraction
When hydrogen runs in a very lean mixture, the flame velocity drops rapidly, slowing combustion and reducing power.
Auto-ignition temperature: Hydrogen resists knocking
The auto-ignition temperature defines when a fuel ignites on its own.
- Petrol: ~230–280°C
- Hydrogen: ~500°C
Hydrogen’s high auto-ignition temperature brings major advantages.
It allows for:
- Higher compression ratios
- Advanced ignition timing
- Strong resistance to knocking
This improves both efficiency and engine durability.
From a combustion point of view, hydrogen behaves like a high-octane fuel.
Energy Density: Hydrogen’s Biggest Weakness
This is where hydrogen struggles the most.
By weight, hydrogen carries enormous energy. That’s why it’s chosen for rockets.
By volume, hydrogen performs poorly.
Inside an engine cylinder:
- Hydrogen takes up about 30% of the space
- Petrol vapor takes up 1-2%
More hydrogen means less oxygen inside the cylinder.
Less oxygen means less power, especially in naturally aspirated engines.
This is one factor that limits hydrogen’s performance potential in regular road cars.
Storage and vehicle design challenges
Hydrogen doesn’t just affect the engine. It affects the entire vehicle.
Hydrogen requirements:
- Requires large, high-pressure tanks
- Strong safety structures
- Complex packaging solutions
Impact of these tanks:
- Reduces boot space
- Increases vehicle complexity
- Makes mass-market car design difficult
Compared to CNG, hydrogen storage requires more compromises.
So, can hydrogen replace CNG?
Technically? Yes.
Practically? Not yet.
Hydrogen combustion engines:
- Eliminate carbon emissions.
- Offer strong efficiency capabilities
- Resist harshness well
But they also face:
- NOX emissions
- Power limitations
- Serious storage challenges
CNG remains easy to store, cheap to implement, and more practical for everyday vehicles today.
Hydrogen is promising, but promises alone don’t translate into mass-produced cars.
Final Verdict: Hydrogen is the Future, but Not a Drop-In Replacement
Hydrogen is not a fantasy fuel. It works. Engineers understand it. Engines can run on it.
But replacing CNG with hydrogen is not a simple swap.
Until storage becomes compact, affordable, and practical, hydrogen combustion will remain a niche solution, not a mainstream replacement.
For now, hydrogen stands as a strong technological possibility, not an immediate alternative.
Sources : sciencedirect.com, omnitekcorp, ngsindia










